How to eliminate many of your cleaning solutions
How to eliminate many of your cleaning solutions
Well, that is a pretty attractive headline, right? Since the average American family of four can expect to pay at least $680 a year for cleaning products, this is a substantial sum of money. (Cleaning Supplies Costs: How to Save Money While Keeping Your Home Clean) How in the world can you get something clean without using a cleaning solution? Water alone can’t clean a surface, right?
If the water is deionized, it can actually clean without any added solutions. If it sounds crazy, you just have to check what’s actually in your water to understand. Normal tap water has minerals such as calcium carbonate (CaCO3) which are left behind when the water evaporates from a surface, leaving water “spots”. The spots are actually the dissolved solids in the water, which can also be ionic.. Ions are atoms or groups of atoms that have a positive or negative charge. For the calcium carbonate example, calcium Ca has a positive charge (2+) while the carbonate CO3 (carbon and oxygen atoms) has a negative charge of 2-. The easiest way to avoid this deposit on your surface (windows, car, etc.) is to remove the minerals from the water.
Here’s where it may help to understand some water chemistry.
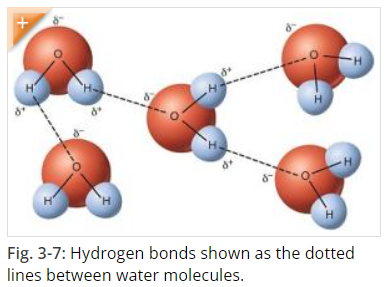
Water, as we know it H2O, is formed of hydrogen and oxygen atoms that “stick” to each other by covalent bonds. Covalent bonds form when two or more nonmetals combine. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds. (Covalent Compounds - Formulas and Names) Hydrogen bonding is responsible for how individual water molecules “stick” to other water molecules, because the hydrogen end of the molecule is attracted to the oxygen of other molecules. However, because hydrogen bonds are weaker than covalent bonds, in liquid water they form, break, and reform easily. (Hydrogen Bonds Make Water Sticky)
Source: Hydrogen Bonds Make Water Sticky
Calcium carbonate, CaCO3, is one of the most common dissolved substances in water. Calcium has a +2 charge, and is considered a metal, and Carbonate (CO3) has a -2 charge and is a non-metal, and the bond between metals and non-metals is called ionic. This is a pretty strong bond, and is one of the reasons CaCO3 doesn’t actually dissolve in water. Because water molecules act like magnets with positive and negative ends, and both the calcium and carbonate ions have electric charges, there is some electric attraction going on between water and CaCO3. The calcium ions hang out with the oxygen part of the water, and the carbonate ions cozy up to the hydrogen part. However, the ionic bond within CaCO3 is stronger than the electric charges between the water and CaCO3 molecules, so the water never really breaks up or “dissolves” the CaCO3. (Is Calcium Carbonate Soluble in Water:Answer and Explanation of Reasons) It precipitates easily, and can be removed from the water solution by several means: reverse osmosis, which is passing the water through very small filters, or by passing the water through positive and negative beds of resin that attract the CaCO3. When the impurities are removed from water, the electric charges that exist on the “ends” of the pure water molecules are freed up again, and water becomes more “sticky”, enabling it to pick up more dirt than water with CaCO3 or other impurities in it. This is why deionized water cleans better than water with impurities in it.
The main enemy of water-based cleaning is Total Dissolved Solids, which are those impurities that get left behind when water evaporates, regardless of whether they were truly “dissolved” in the water to begin with. Car enthusiasts, window washers and solar panel cleaners are big fans of DI water. Why? It has no dissolved solids, so it leaves no spots or impurities behind when it dries. Spots are unsightly, and when left to bake onto a car’s finish in the sun, can actually “etch” the clear coat of the car.. Now, many car detailers will wash their cars with normal car-washing liquids and water, but save the DI water for the final rinse. This conserves DI water, which can be pricey because of the filtration process. Many professional window cleaners and solar-panel cleaners, however, use it exclusively (no chemicals) with soft brushes, so that the cleaning process from start to finish relies on DI water. Remember, windows and solar panels are big areas that are continually baked in the sun, so washing them with normal soap and water and then rinsing with DI water can cause the soap to bake on before you even get to the rinse step! They are also very-high visibility surfaces, and spots on solar panels can translate into big efficiency losses, which are a large reason they are installed to begin with.
True deionizatIon systems can cost a lot. For the average person wanting to clean their home with less chemicals however, using deionized water is not out of reach. It all depends on how you get it and how you use it!
Before we get into home deionization systems, however, you’ll want to understand the difference between distilled water and DI water. Water distillation is a physical process where the water is boiled and the steam condensed for purification. Although distilled water has less total dissolved solids than tap water, it’s still not free of impurities like DI water. That said, both deionized water and distilled water are safe for human consumption as long as there are no germs (viruses, bacteria, cysts), which may pass through the deionization process because these are generally not charged (ionized) particles. DI water is also not the same as “softened” water: softened water still has TDS in it and water softeners also do not remove bacteria and viruses.
That said, there are some home systems (like ZeroWater) that can produce deionized water that is safe for consumption and better for cleaning. Because municipal water systems already employ disinfection systems like chlorination to kill bacteria and viruses, using ZeroWater filters with city water is sanitary AND free from TDS, because the ZeroWater filter removes TDS. ZeroWater filters are 5-stage filters. One of the stages is a mixed bed (cation & anion resins) of small sized polymeric beads. Ion exchange is a reversible chemical reaction where dissolved ions are removed from solution and replaced with other ions of the same or similar electrical charge. The cation beads contains hydrogen ions (positive charge). The anion beads contain hydroxide ions (negative charge). The resin works by exchanging contaminant ions in the water with the hydrogen and hydroxide ions.The contaminants attach to the beads while the hydrogen and hydroxide are released into the water. These two ions combine together to produce H2O. (How does Ion Exchange Technology Work?)
Reverse Osmosis (RO) is another type of system that deionizes water. There are many different manufacturers of RO systems, and if you have one, you’re probably very satisfied with the quality of water. RO removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable membrane to remove dissolved solids. After water exits the RO membrane, it passes through a postfilter to polish the drinking water before it enters a dedicated faucet. RO does not remove some types of bacteria and viruses from the water, though, so this is why it should be used with municipal water or another disinfection mode, like UV light. (What Is a Reverse Osmosis System and How Does It Work?)
Therefore, if you have ZeroWater or RO water, you can test the water for TDS (many come with an included TDS meter) and use it as deionized water for cleaning. Since filters do have a cost, however, you’ll want to maximize the use of your deionized water. For example, a 4-pack of ZeroWater filters is $55, which is about 92 cents per gallon of water, whether you use it for drinking or cleaning. Here are our suggestions:
Use DI water for spray-bottle applications where you mix your own cleaner (like TotalClean)
Use DI water for vacuum mops/steamers so floors get cleaner and look better
Use DI water as a “rinse” for areas where water spots are most visible
Use DI water for “descaling” modes of small appliances if water is called for (like coffee machines, baby-bottle sterilizers, steam irons and other heated appliances)
Unfortunately, larger-scale DI systems used for washing cars, windows and solar panels can incur substantial cost not only in equipment but in maintenance, as they require replacement of the resin beads periodically as they become fouled with minerals. Beware of cheap In-line water deionizers like this one, reviewed in this video, however, because they don’t work well in areas of water with high TDS.
CR-Spotless Water Systems - DIC-20, $438, has 2 mixed-bed resin beads, a moveable cart, and a battery-operated TDS meter. The reservoir should produce 300 gallons of deionized water, so theoretically the 2 replacement cartridges for $139 should give DI water at a cost of 47 cents per gallon.
According to this car detailing video, this On The Go dual-bed system, $600, is the best budget system if you plan on using at least 1200 gallons per year. Now, this is a lot of DI water, but if you like to wash cars, boats, windows, showers, solar panels, etc., this could be realistic for you at about 25 cents per gallon for the refills.
Well…is deionized water a draw for you? Maybe not, if you don’t have an abundance of cars, boats, etc, but the ZeroWater filter is a nice all-purpose drinking water filter that also provides deionized water for a small amount of spot-free cleaning. If you have basic home cleaning needs, there are other ways to remove minerals and contaminants from your water. Check out our article Non-Toxic Ways to Deal with Hard Water for more of them!
Photo by Andres Siimon on Unsplash