Non-toxic ways to deal with Hard Water
Non-toxic ways to deal with Hard Water
Many of us live in areas with very “hard” water. How can water be hard? It’s a way of saying there are a significant amount of minerals in the water, which can leave spots on your appliances, clog your pipes or leave a filmy residue on your hair or skin after showering. If, despite frequent cleaning, your toilet looks like the following, you probably have hard water!
Hard water is not necessarily bad for you; after all, many “mineral waters” for consumption capitalize on these very minerals that we are not fond of looking at on our appliances.
According to the science, “hard” water can be categorized into alkaline (e.g., calcium carbonate CaCO3), non-alkaline (e.g., CaSO4), and silica based, with alkaline being the most common. This water chemistry will of course affect the ability to prevent scale.
Since CaCO3 is the most common type of mineral, most findings are delivered as a number that reports the concentration of calcium carbonate or calcium carbonate equivalents for a given unit of water. This result may be expressed in grains per gallon (gpg), parts per million (ppm), or milligrams per liter (mg/L). According to the Water Quality Association, the hardness scale, measured in gpg of calcium carbonate, can be represented as follows:
Less than 1 gpg is considered soft
Between 1 and 3.5 gpg is considered slightly hard
Between 3.5 and 7 gpg is considered moderately hard
Between 7 and 10.5 gpg is considered hard
More than 10.5 gpg is considered very hard
(Source: How is water hardness measured?)
If you want to “soften” your water, there are many solutions ranging from a few dollars to thousands of dollars, and from chemical-free to lots of chemicals. Obviously, the best would be chemical-free and cheap…but most preferably chemical-free. Here are 3 proposed solutions to keeping minerals from adhering to the surfaces your water comes in contact with (shower doors, bathtubs, toilets, sinks, etc.). You can:
- Add a true water “softener” into your water source to remove the minerals
- Add a slick “film” or coating to the appliances so that the minerals don’t stick.
- Change the chemistry of the water so that the minerals don’t stick (EMF, MWT, AMT)
Let’s dig into these to find out which is best for you. Our first recommendation is to test your water. There are a lot of water testing kits on the market, some of which have 16-20 functions (a lot of tiny colors on the strip!) While these are good for getting an overall picture of water quality, if you are interested in hardness, a specific test for hardness has better clarity. This one by Varify retails for $12 on Amazon. If your water comes in more on the green side (low minerals), you may need to do more research on the nature of your water “stains” because that result shows it’s actually low in minerals.
If your water shows as moderately to very hard (above 3 grains per gallon), then you might want to do something about it. Moving on to our 3 proposed solutions:
Water Softeners
Softener systems actually remove minerals such as calcium and magnesium from your water by using an “ion exchange” process. The softener passes incoming water through a bed of resin beads, where minerals are attracted to the beads and softened water flows out of the tank. Once in a while, the beads must be regenerated by flushing it with a strong solution of sodium chloride (salt) or potassium chloride, causing the minerals to release from the beads. Then the system is ready to soften water again. Softeners come in different sizes, for “whole-home” or smaller “appliance” use. Although the upfront cost is more, the per-gallon cost is typically lower on whole-home systems. In addition, appliances all over your home, from your coffee maker to your washing machine perform better with softer water. However, there are disadvantages to using whole-home softeners: they can corrode pipes (it’s not recommended to soften water on very new pipes; you’ll want to wait several weeks to months so that an internal mineral film will develop), it does add a small amount of sodium to your drinking water, and regular testing of the water and maintenance of the softener is necessary to make sure the softener is working properly. (Home Water Softening Frequently Asked Questions) Since there are many whole home systems available, we chose to review a few systems that soften specific appliances where people see the most impact.
Water softeners also lower the surface tension of water, making it feel “wetter” or “more slippery”. On a porous surface, having a lower surface tension allows water to penetrate deeper allowing for better cleaning. The addition of soap or the use of hot water will both lower the surface tension of water… Water softeners function through the process of ion exchange, i.e. exchange calcium and magnesium ions for sodium ions. The conclusion can be drawn that sodium lowers the surface tension of water while calcium and magnesium ions increase the surface tension…There are other factors that influence the "wetness" or "slippery" feel of soft water including pH and alkalinity. Typically the higher the alkalinity and pH, the greater the impact of this phenomenon. This may help to explain why naturally soft water or reverse osmosis water do not have the same "wetness" or "slippery" feel. (The Kinetics and Aesthetics of Soft Water)
Softeners are measured by the number of “grains” they remove before regeneration is needed. Here’s where you need to know how hard your water is (hence testing is needed!). For example, if your water is 10 gpg, there are 3 people in your household and since the average person in the US uses about 75 gallons of water per day, that means 10 x 3 x 75 = 2,250 grains per day. A water softener is usually sized to regenerate about one time per week, so that means a softener of 15,750 grains would work (16,000 grains like this one is ideal for an RV or live-aboard boat). This article has very good information about the salt efficiency of different softeners, as using a lot of salt to regenerate is not only costly, it’s not good for the environment
Washing dishes and clothes in hard water doesn’t yield great results. Just like the inside of the sink or toilet, minerals can build up on your washed clothing over time, making them feel stiff and look dingy. In the dishwasher, minerals deposit on dishes causing spots and incomplete rinses. “Water softening tablets” are available for use in the laundry or dishwasher and here are some non-toxic brands that work well with hard water:
- Calgon 4 in 1 Water Softening Tablets, $51, for 75 tablets for laundry washing machine: add 1 tablet with each load of laundry (and use your own detergent). Mainly composed of polycarboxylates, these tablets prevent minerals from depositing on surfaces and are generally deemed safe for human contact (after rinsing) and the environment. Therefore, although these are “chemicals”, you can safely add softeners to your dishwasher or laundry:
- BioKleen Free & Clear Natural Laundry Detergent, $35 for 150 loads, is very low cost for a natural detergent and is said to work well for moderately hard water. Like many non-toxic detergents, it dissolves and works better in warm water rather than cold.
- Planet Automatic Free & Clear Dishwasher Pacs, $6 for 20 pacs, is among the lowest cost per load, has transparent ingredients, and is good for hard water according to reviews.
- Blueland Dishwasher Detergent Tablet Starter Set, $30 for 60 tablets, do not have wrappers at all and are good for hard water according to reviews.
Shower “filters” are a great idea to prevent the harsh chemicals that municipal water treatment companies employ to keep drinking water safe. Mostly we’re talking about chlorine and its by-products (see our article about the nasty effects of using too much chlorine). But most shower filters don’t address hard water (which causes all those spots on your glass shower doors), or iron or sulfur in the water. However, there are some shower filters that do; you must read the product description carefully to see what is removed. The first product below is primarily a water softener to remove hard water minerals; the next 2 products are filters with some water softening capabilities.
- ShowerStick Shower Water Softener, $260: This company has done its homework on water softening and actually allows the customer to do so as well, by providing a water testing kit with their kit. Using the water test weekly will show you when to “regenerate” the resin beads inside, which accomplish the softening. Depending on how often you use the shower, regeneration may need to be done on a weekly basis. The company also offers a KDF water filter to remove 95-99% of chlorine and controls the buildup of microorganisms such as bacteria, algae, fungi and mold.
- PureAction Water Softener Shower Head Filter for Hard Water, $40, is a shower head meant to replace your existing shower head. It comes with 2 extra filters that are replaceable (the filter cartridge is what removes the minerals and chlorine). According to reviews, customers with sensitive skin have had good results with this showerhead.
- AquaEarth 15 Stage Shower Filter, $30, is an in-line water filter that allows you to add your own shower head. It lasts approximately 6 months and replacements run about $7.50 each ($30 for a pack of 4).
Coatings that inhibit scale formation
There are a lot of anti-scale coatings available for commercial equipment, but not so many for residential use. The application of a coating is sometimes not so difficult when a fixture is new (like a new toilet), but doing the necessary cleaning and application in an older fixture can be a lot of work. In addition, the chemicals that make surfaces slick enough to inhibit scale are often not disclosed. For example, Spotless Toilet Coating contains 84-94% isopropyl alcohol (for quick drying) and 0.5-1% of a proprietary acid, leaving 5-10% undisclosed ingredients. (MSDS)
Salt-free Water Conditioners
There are a number of water “conditioners” that do not use salt, electricity, or other energy to keep minerals from depositing in your appliances. Here is a rundown of these technologies:
- Template-Assisted Crystallization (TAC) uses surface-treated resin beads to convert (not remove) dissolved hardness ions to microscopic scale-resistant crystals. The polymeric beads are fluidized, creating agitation that releases the microscopic crystals and allows for further formation of crystals. Once these crystals are formed and released from the beads, they are insoluble particles that do not form scale on surfaces. In some cases, a fine dust may form on dishes but it can be wiped away. Template-assisted crystallization systems typically require relatively clean water as the input, and may require pretreatment if the water contains high iron and manganese concentrations or other sediment..(Drinking Water Treatment Salt-Free Water “Softener” Options) Brand names include Aquasana, AO Smith and Pentair-Pelican.
- Ultrafiltration and Nanofiltration: These processes use very fine filters to remove bacteria, viruses, and some salts from water. This article by the Safe Drinking Water Foundation shows the different substances these processes can remove.
- Reverse Osmosis: These systems work by pushing water through a microscopically small filter material. This semi-permeable membrane has a pore size of around 0.0001 microns, effectively only allowing the small water molecules through and catching any larger molecules of contaminants, organic materials or even salt. Originally designed to desalinate seawater and reduce high chemical contaminant material such as heavy metals, reverse osmosis is now in use in many government, commercial, military and even residential applications. It does produce ultra-pure water, but also wastes a lot of water due to back-flushing requirements, and is relatively expensive. (Learn The Pros And Cons Of Reverse Osmosis Water Filtration Systems)
Magnetic Water Treatment, Anti-Scale Magnetic Treatment, Electromagnetic Fields
There’s been a fair amount of studies on AMT (anti-scale magnetic treatment) or MWT (magnetic water treatment) or EMF (Electromagnetic Fields). Wikipedia states that it is “unproven and unscientific.” However, EMF has seen a lot of study since 2010, and one meta-study concluded that although different results were reported regarding the influence of EMF in minerals precipitation, the results support the same hypothesis that EMF induce bulk precipitation of crystals rather than adhesion to the surface of reactors, pipes and vessels or to membrane surfaces. If we consider the bulk precipitation enhancement as effective EMF treatment, the percentage of effective EMF cases can reach 95% for the discussed 48 studies, 5% of the studies observed negligible improvement with EMF treatment, none of them has negative results. This is not “unproven and unscientific.”
There are two configurations of an EMF device used in water systems: permanent magnet and solenoid coil (uses alternating or direct current). The efficiency of EMF depends on the properties of the field, including intensity, waveform, and frequency (the field strength varies with the number of coils or the thickness of the wire used), the material of pipe or surface, pH of the water, temperature of the water, residence time (how long the water is exposed to the EMF), and suspended particles (in some cases the presence of suspended particles such as silica is necessary for EMF water treatment to be effective, which can adsorb metal ions and increase bulk precipitation). (EMF meta-study)
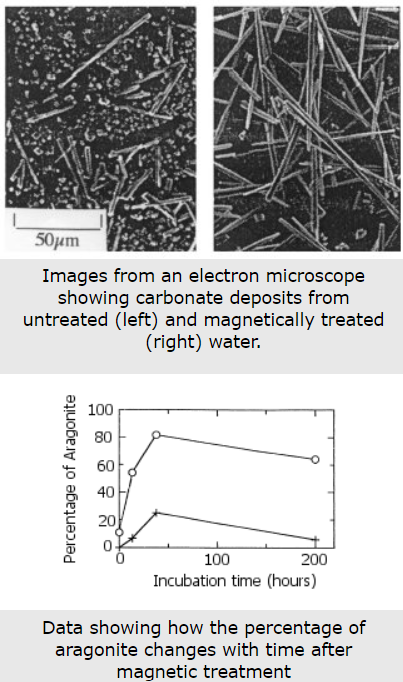
Under ordinary circumstances, the scale forms through heterogeneous nucleation of CaCO3 (calcium carbonate) on the substrate surface. By contrast, when magnetically treated is used, nucleation (formation of crystals at a molecular level) takes place homogeneously in the body of the water, and small disc-shaped crystallites (about 50 #m in diameter and 1 #m thick) are formed. Initially suspended, these crystallites gradually settle as a sediment at the bottom of the container. (Magnetic Treatment of Water: A Theoretical Quantum Model)
When magnetic water treatment was first patented in 1945 by a Belgian company, there was not a complete understanding of how magnetic fields inhibited scale formation. Today, however, one theory is presented here: “Through the efforts of universities and their extensive laboratories, the performance of the magnetic water treatment for scale prevention has moved from being a phenomenon to understanding that the magnetic field creates a hardness crystal called Aragonite. It forms this because a tiny percentage of water is always dissociating – hydrogen (proton) leaving and forming H3O or hydronium – and the energy imparted to the water by the magnet causes the percentage of hydronium to increase dramatically. Water missing the hydrogen reacts differently with calcium bicarbonate (calcium hardness) than does water with full hydrogen in the size, shape, and texture of calcium carbonate crystals formed as evidenced in electron microscope photos. All crystals are void of charge so they won’t adhere to metals, however, the aragonite form is softer and is easily flushed through plumbing. No magic and no mystery. The performance relative to scale prevention is directly proportional to magnetic field strength and speed of water through alternating magnetic fields.” (Magnetic water treatment for scale prevention)
This is similar to the explanation given in a paper from 2000: MWT changes the form of calcium in water. The researchers tested MWT by passing water through a magnetic field of 1000 Gauss (0.1 Tesla). The samples were then heated in open beakers, forming scale when the water evaporated. The scale was inspected by X-ray diffraction (which can reveal what it’s made of) and an electron microscope (to view the structure).
The results confirm earlier claims that there are two different types of calcium deposits made: calcite and aragonite. They are both made of the same stuff (calcium), but form in different structures. The small beads of calcite tend to make hard scale that clings well to surfaces. Aragonite forms in longer shapes which are less prone to form hard scale, and keep moving along with the water. The data they collected also confirms that the effect can last over a period of time, as much as 200 hours.
Source: Magnetic Water Treatment, K&J Magnets, Inc.
In conclusion, if you have calcium carbonate in your water, then MWT may work in preventing some scale buildup. Since it’s likely that many small magnet systems are not strong enough for the amount of water flow, it’s best to purchase from a company that knows its science (and offers different size magnets/appliances for different size pipes).:
- Magnation: this company employs several technologies, not just magnets, into their products. They have a questionnaire enabling you to find the right product.
- ESF scale preventer uses permanent magnets, but you need to install them in-line with the water pipe, which may require a plumber. (contact company for price)
- Build your own: Using K&J’s equations, they have calculated the strength of the magnets necessary to do the work, and they sell them! Basically you just have to measure the diameter of the inlet pipe where you are going to place the magnets, and build a system to place them opposed over the pipe so they don’t fall off or slam into each other. They offer magnets in strong, stronger and strongest energy.
One more product was tested by one of our team members. Krazy Klean is a magnet-based product that is placed in the toilet tank to reduce scaling in the toilet bowl, leading to less cleaning and use of chemicals. In the toilet we tested, it definitely worked. Old deposits were not removed, but once the bowl was cleaned (see our article here for non-toxic methods), it stayed clean for a month test period (from waste and minerals) with the Krazy Klean device in the tank, whereas it was previously cleaned about 2x per week yet still had waste and mineral residues building up. The manufacturer advertises "Just drop it in your tank and eliminate scrubbing for an entire decade", however, we promote cleaning your toilet bowl regularly with non-toxic cleaners to reduce germs. The company provides a report of its scientific testing on its website if you'd like to check out how it works.
Photo by Andres Siimon on Unsplash