What are xenobiotics and POPs and how do our bodies deal with them?
Xenobiotics surround us everyday! If you have an illness that you can pinpoint to a chemical or environmental exposure, then you know what a xenobiotic is and how it can seriously affect your health.
Xenobiotics have been defined as chemicals to which an organism is exposed that are extrinsic to the normal metabolism of that organism. (Progress in Molecular Biology and Translational Science). Since mold produces mycotoxins that are not made in our own bodies, these mycotoxins are xenobiotics to us, as are many man-made chemicals like POPs. (Alcohol is also a xenobiotic).
Persistent Organic Pollutants (POPs) are chemicals of global concern due to their potential for long-range transport, persistence in the environment, ability to bio-magnify and bio-accumulate in ecosystems, as well as their significant negative effects on human health and the environment. The most commonly encountered POPs are organochlorine pesticides, such as DDT, industrial chemicals, polychlorinated biphenyls (PCB) as well as unintentional by-products of many industrial processes, especially polychlorinated dibenzo-p-dioxins (PCDD) and dibenzofurans (PCDF), commonly known as dioxins. (Food safety: Persistent organic pollutants (POPs)) POPs are fat-soluable, and tend to accumulate in our fat tissues. POPs are xenobiotics, but not all xenobiotics are POPs. Exposure to POPs has been associated with diabetes, cardiovascular diseases and many other chronic diseases. (Glutathione!)
Most of these xenobiotics are transformed by enzymes in the liver, and are then eliminated by excretion. First of all: What is an enzyme? Enzymes are complex proteins produced by living organisms that act as catalysts in chemical reactions. Enzymes can either build up or break down. Enzymes themselves are not consumed.
This is where our genes come in. “GST” genes are important for detoxification of the body, in that they manufacture those enzymes that facilitate the detoxification reaction. One of the most important GST enzymes is GSTP1.
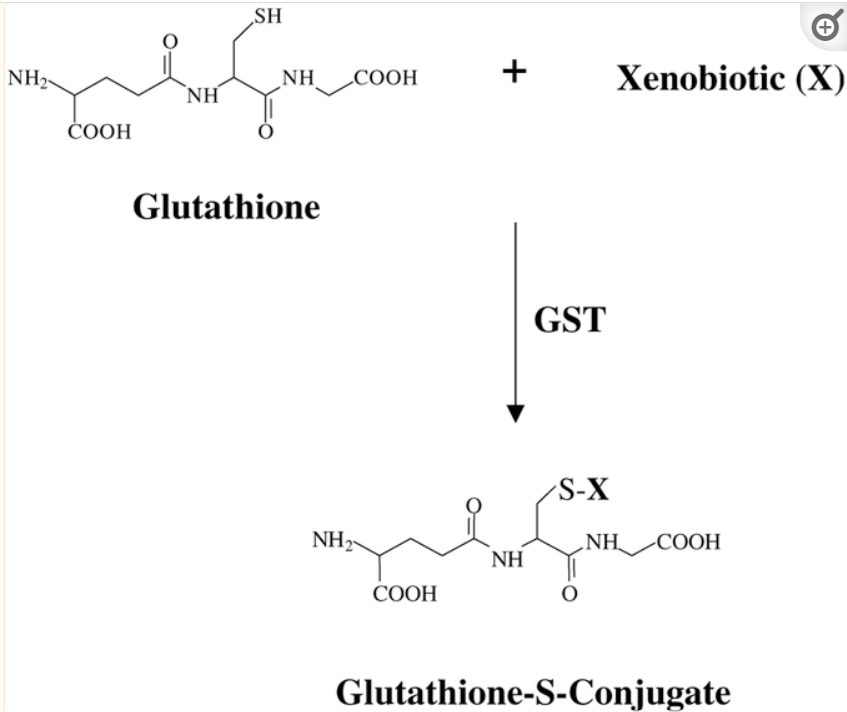
The GST pi gene encodes (provides instructions for building) the enzyme Glutathione S‑transferase Pi (GSTP1), which plays an important regulatory role in detoxification, anti‑oxidative damage, and the occurrence of various diseases. The detoxification reaction is called “glutathione conjugation”. (GSTP1 and cancer: Expression, methylation, polymorphisms and signaling (Review)) Following is an example of glutathione conjugation; note that the “SH” site on glutathione (sulfur) is the binding site for the xenobiotic:
Source: The role of glutathione-S-transferase in anti-cancer drug resistance
GSTP1 has a wide range of physiological functions: It is involved in metabolism, detoxification and elimination of potentially genotoxic foreign complexes, metabolizes a variety of carcinogenic compounds, and protects cells against DNA damage and canceration. However, while GST mediates detoxification from accidental xenobiotics, like exposures to pesticide for example, GSTs have also been implicated in the development of resistance toward chemotherapy agents, especially platinum-based chemotherapy drugs. (GSTP1 and cancer: Expression, methylation, polymorphisms and signaling (Review))
Here are several more genetic terms that will help to understand how GST and GSTP1 work:
Gene expression is how GST directs the manufacture of GSTP1 (for more on how gene expression works, check out this article)
Methylation is a chemical modification of DNA and other molecules that may be retained as cells divide to make more cells. When found in DNA, methylation can alter gene expression. In this process, chemical tags called methyl groups attach to a particular location within DNA where they turn a gene on or off, thereby regulating the production of proteins that the gene encodes. (National Human Genome Research Institute)
Polymorphism, as related to genomics, refers to the presence of two or more variant forms of a specific DNA sequence that can occur among different individuals or populations. The most common type of polymorphism involves variation at a single nucleotide (also called a single-nucleotide polymorphism, or SNP). Other polymorphisms can be much larger, involving longer stretches of DNA. (National Human Genome Research Institute)
GSTP1 methylation can affect gene expression, inactivating the GST gene. GSTP1 methylation has been associated with the development or recurrence of prostate cancer (PCa), liver and breast cancers.
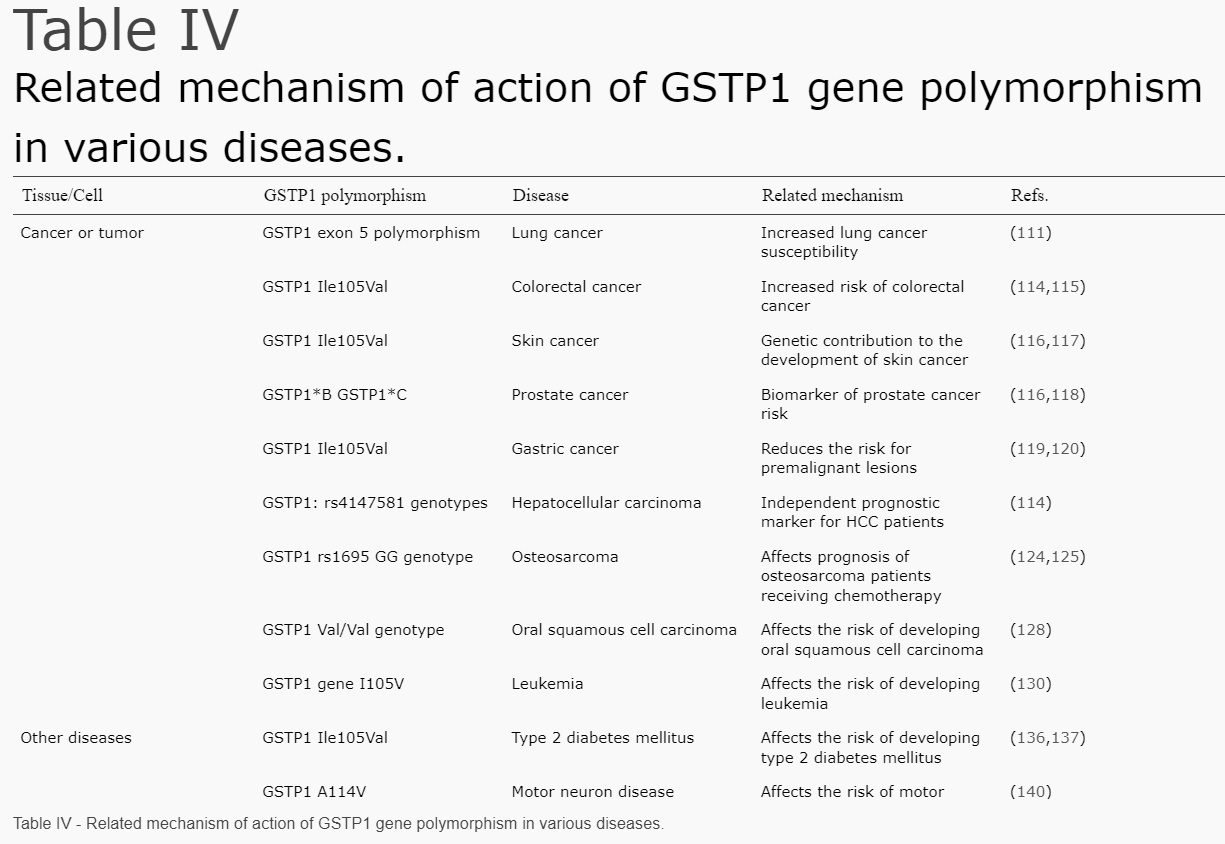
In addition, during detoxification of xenobiotics, GSTP1 may become damaged, causing polymorphism (a variation). Some polymorphisms are associated with specific cancer types. For example, the genetic polymorphism of GSTP1 may be associated with the detoxification of polycyclic aromatic hydrocarbons (PAHs) in cigarette smoke and exhibits the highest expression in lung tissue. More polymorphisms of GSTP1 and associated cancer risks are shown in the next table:
Source: (GSTP1 and cancer: Expression, methylation, polymorphisms and signaling (Review))
Therefore, xenobiotics can not only overload the GST detoxification processes, but they can damage GST and the enzymes it encodes, like GSTP1. Restricting xenobiotics and stress is crucial to keeping this important defense system working optimally!
Here’s a bit more on how these genes detoxify. Although GST genes do not make glutathione (GSS genes do), they regulate its use.
Glutathione has been described as “the mother of all antioxidants” because it recycles vitamins C and E, which are other antioxidants, and of course it binds and modifies toxins from our environment so that we can get rid of them. According to Dr. Mark Hyman, “The secret of its (glutathione’s) power is the sulfur (SH) chemical groups it contains. Sulfur is a sticky, smelly molecule. It acts like fly paper and all the bad things in the body stick onto it, including free radicals and toxins like mercury and other heavy metals.” (Glutathione: The Mother of All Antioxidants)
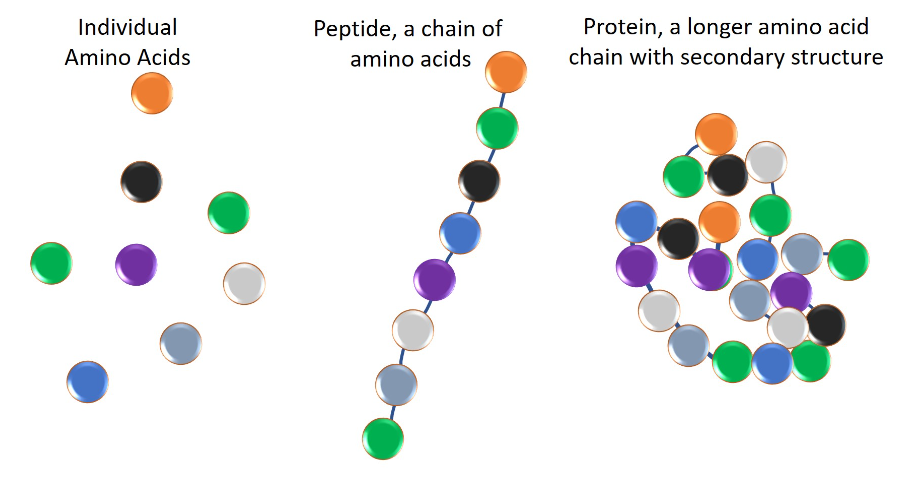
Glutathione (GSH) is a tripeptide molecule consisting of the amino acids glutamate, cysteine, and glycine. It is the most abundant antioxidant in the human body that contains thiol (an organic sulfur compound). Peptides are chains of 2 to 50 amino acids that are linked together. For reference, proteins are also chains of amino acids linked together, but these number over 50 and usually more than 100. Here is a visual aid, noting that Glutathione falls under the Peptides category.
Source: The Difference between Peptides and Proteins
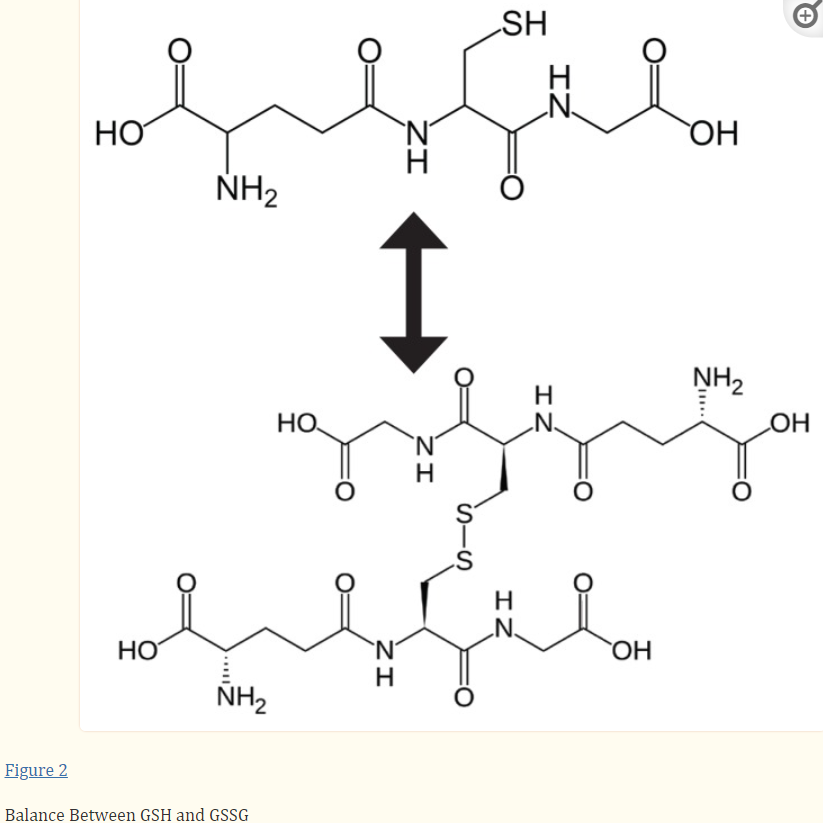
Glutathione exists in two states in cells: reduced (GSH) and oxidized (GSSG). Oxidized glutathione is actually 2 reduced glutathiones bound together at the sulfur atoms.
Source: Glutathione!
The difference between reduced and oxidized glutathione is that GSH (reduced) is the “recharged” version, while GSSG (oxidized, also called glutathione disulfide) is the “spent” version. GSH is also called “free glutathione” in that it has its sulfur site ready to bind to xenobiotics, while the site on the spent version is not available because it’s stuck to another glutathione molecule.
The body is constantly recycling glutathione from the oxidized to the reduced version (for more on how this happens, this video is really helpful) . Healthy cells at rest have a GSH/GSSG ratio >100:1, meaning that there is much more GSH (recharged) available than GSSG (spent). When cells are exposed to oxidant stress such as xenobiotics, the ratio can drop to 1:10. This is very dangerous, because depletion of GSH and accumulation of GSSG is actually directly toxic to cells, causing their death (apoptosis). (Glutathione!)
Glutathione (GSH) production also drops with age and disease. Unfortunately you can’t just “pop a pill” for more GSH (contrary to many medical claims on the internet!). The body, however, can make its own GSH in the liver with the amino acids cysteine, glutamate, and glycine. The best ways to boost our bodies’ manufacturing of GSH are to eat foods rich in glutathione or its building blocks (amino acids of cysteine, glutamate, and glycine), increasing your intake of vitamin C, and getting enough sleep and exercise. (10 Natural Ways to Increase Your Glutathione Level)
Here’s a recap about GST, GSTP1, and glutathione:
GST is the gene responsible for encoding GSTP1.
GSTP1 is the enzyme that regulates the ability of glutathione to bind to xenobiotics.
Glutathione is an an enzyme that exists in 2 forms: GSH (reduced) and GSSG (oxidized).
GSH is also called “free glutathione” and in healthy cells, exists in a 100:1 ratio with GSSG.
Xenobiotics are those chemicals to which we are exposed that come from outside our bodies. POPs (persistent organic pollutants) are xenobiotics.
GSH binds with xenobiotics in the presence of the GSTP1 enzyme.
GSSG cannot be used to bind xenobiotics, it first must be converted back to GSH.
Stress and xenobiotics are dangerous in that they can cause changes to GST and GSTP1 which affect their ability to detoxify our bodies, making the body prone to cancer.
Unchecked stress and xenobiotics also overwhelm free glutathione, causing cell death.
We can help restore proper glutathione balance and immune function by limiting stress and xenobiotics, eating the right foods for manufacture of GSH and getting enough sleep and exercise.