Do HypoAir products kill the “good” bacteria as well as “bad” bacteria?
Do HypoAir products kill the “good” bacteria as well as “bad” bacteria?
Short answer: yes, some good bacteria are killed, but let us explain a little about the nature of bacteria, and how this technology affects them!
Since HypoAir’s bipolar ionization is made for the home, we are talking about “good” bacteria for humans, found on exposed home surfaces, the skin, and upper respiratory tract, because this type of ionization does not penetrate to interior surfaces.
So the answer is: yes, bipolar ionization does kill some “good” bacteria, but the type of bacteria, on which surfaces, at what humidity, at what concentration of ions, and so on, are highly variable! We find that the biological and air quality contaminants found in homes are typically in high unhealthy concentrations, which are typically not found in the outside air. We want to reintroduce natural counterbalances to suppress the spread and growth of these biologicals indoors, to make them more similar to what's found in nature. However, our technologies are not going to sterilize the environment; they're just designed to cut concentrations and reduce illness in families. In 20-30 years, technologies like ours could become very cost effective and installed throughout a home to have a nearly sterilizing effect in our indoor environments. We don't want that! At that point, the intentional reintroduction of a positive biome would be advisable. If you are concerned that the use of bipolar kills too many good bacteria, you may want to investigate probiotics for the air to replace those good bacteria on surfaces, and use gentle cleansers and soap for your skin, dispensed from containers that don’t promote the growth of bacteria. And, consider the fact that pets (and dogs especially) vary the nature of your home’s microbiota a lot too!
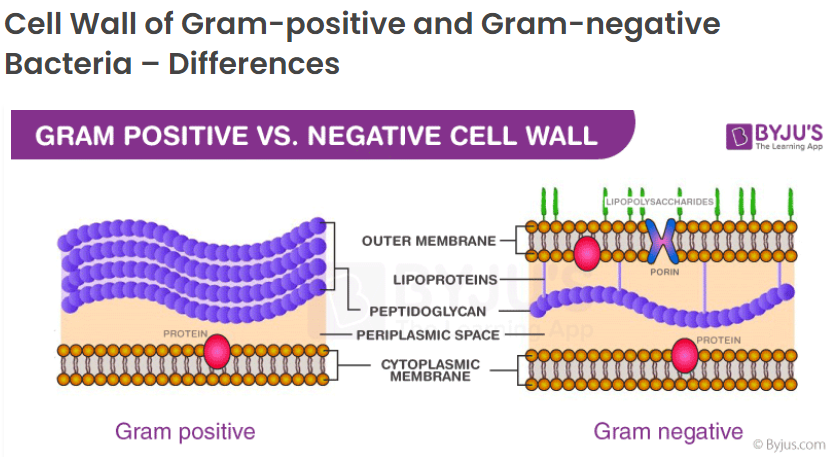
Getting back to bacteria, here’s a short refresher from an article about bacteria, endotoxins and exotoxins: bacteria can be classed into two different groups: “Gram-negative” or “Gram-positive”. These classes are based on a test developed by scientist Christian Gram in 1884, which differentiates the bacteria using a purple stain. According to webmd.com, bacteria either have a hard, outer shell, or a thick, mesh-like membrane called peptidoglycan. The hard outer shell will resist the purple stain, and show up as a red color. These are called “gram negative” because the purple stain did not show. Bacteria with the peptidoglycan absorb the purple stain much more easily and are called “gram positive”. The stain also tells many more characteristics about the bacteria and the way it interacts with bipolar ions.
Bipolar technology is also called cold atmospheric-pressure plasma (CAP), or non-thermal plasma (NTP). In a study which analyzed how plasma affected bacteria in soil, it turned out that the non-treated soil consisted of both gram-positive and gram-negative bacteria from different phyla (a level of classification). After treatment with plasma, however, the gram-negative bacteria were mainly eradicated, and only the major phyla of Firmicutes (gram-positive) were left. Presumably this has to do with the structure of the bacteria.
The authors cited two previous studies on treatment of E. Coli (gram-negative) and S. Aureus (gram-positive) with cold plasma. In the first study, the treated Gram-positive bacteria was mainly inactivated by intracellular damage, while the Gram-negative bacteria expired mainly by cell leakage. The second study showed that plasma treatment led to damage of the bacterial cell wall of both E. coli and S. aureus and a decrease in the total concentrations of nucleic acid and cellular protein. However, S. aureus (gram positive) was less susceptible to plasma exposure in comparison to E. coli (gram-negative).
The sum of these three studies seem to indicate that gram-positive and gram-negative bacteria are affected by plasma differently, and chances of survival of bacteria after treatment with cold plasma is higher if a bacteria is gram-positive, having more of the mesh-like membrane (peptidoglycan). One can see from the diagrams below that these peptidoglycan layers are relatively thick on the gram-positive type, which may account for its resistance to plasma. Depending on the relative humidity of the air, plasma can form varying quantities of reactive oxygen species such as hydroxide ions (OH-), hydroxyl radicals (•OH), atomic oxygen (O), hydrogen peroxide (H2O2), and singlet oxygen (1O2). Ozone (O3) is another ROS formed by plasma generators, however we’ve excluded it from HypoAir ionizers by limiting the input energy. These ROS are reported to damage the bacterial structure and functions. In addition, the multiple reactive nitrogen species (RNS), including nitric oxide (NO), peroxinitrites (ONOO−), nitrites (NO2−), and nitrates (NO3−), can play a major role in the plasma’s biocidal process by altering the cell wall components, the functions and the structure of the phospholipid bilayer, the structure of nucleic acids and cellular proteins, gene expressions, and protein synthesis. (Effects of Atmospheric Plasma Corona Discharges on Soil Bacteria Viability)
Image source: Difference between gram-positive and gram-negative cell wall
However, there are factors other than gram-type that affect bacterial eradication via plasma technology, such as pH, humidity, and the surface on which the bacteria were placed during plasma exposure. Specifically,
- Lower pH can translate to higher kill rates. A reduction of 4.9 log was observed when Bacillus cereus was treated at pH 5, while a reduction of only 2.1 log was observed at pH 7. Interestingly, the same study showed that “No appreciable differences between gram-positive and gram-negative pathogens were observed, although the spore-forming B. cereus was more resistant to plasma than non-spore-formers.” (Spores in bacteria are not the same as mold spores; only one bacteria makes one spore).
- Humidity was also reported as an important parameter; increasing the relative humidity was correlated to efficiency in plasma inactivation of Aspergillus niger, which was explained by the generation of more hydroxyl radicals. However, the same study showed that “In contrast, B. subtilis showed slightly poorer inactivation at high gas humidity.”
- Regarding the surface on which the bacteria were placed during plasma treatment, higher eradication was observed when microorganisms were loaded on a filter compared to a fruit surface, because the microbes could “migrate” to the interior of the fruit. Therefore, if the bacteria could migrate into a moist surface, it was more likely to survive. (Cold Atmospheric Plasma Disinfection of Cut Fruit Surfaces Contaminated with Migrating Microorganisms) Wow, bacteria can migrate!
Now that we know that there are a lot of variables in your home that affect the mortality of bacteria, how likely is it that “good” bacteria on skin, your upper respiratory system, and home surfaces will be killed?
First of all, let’s look at what types of bacteria these are. Staphylococcus epidermidis (phylum Firmicutes, gram-positive) is a part of the skin microbiota (aka skin flora) and another type of good bacteria is Roseomonas mucosa (phylum pseudomona dota, gram-negative), which is naturally present on the skin and contributes to an overall healthy skin microbiome. (Dermatologists Break Down the Difference Between Good and Bad Bacteria) In addition, the optimal pH value of skin on most of our face and body lies between 4.7 and 5.75, which is mildly acidic. (Understanding skin – Skin’s pH) According to the studies above, it’s not known whether good bacteria on healthy skin survive plasma treatment, because although healthy skin is normally mildly acidic (which promotes their death by ions), moist skin favors preservation of good bacteria. Therefore, no matter what relative humidity is in your home, it’s a good idea to keep your skin hydrated!
Concerning the upper-respiratory tract, potential keystone microbiota are Dolosigranulum and Corynebacterium species (both gram-positive), as they have been strongly associated with respiratory health and the exclusion of potential pathogens, most notably Streptococcus pneumoniae, in several epidemiological and mechanistic studies. (The microbiota of the respiratory tract: gatekeeper to respiratory health) Regarding pH, airway surface liquid pH in normal airways ranges in vivo between 5.6 and 6.7 in the nasal mucosa, and is around 7.0 in bronchia. (Airway Surface Liquid pH Regulation in Airway Epithelium Current Understandings and Gaps in Knowledge) Therefore it’s mildly acidic in the upper regions, and tending toward neutral pH in the lower regions. Being gram-positive favors survival, as does being in mucous, but being on a mildly acidic surface favors eradication of these good bacteria. Again, keeping your mucous membranes moist via water intake and plain saline sprays is a good idea!
Finally, most of the ions that are emitted by bipolar devices will contact surfaces in our homes. What kind of good bacteria live on surfaces? Forty homes in North Carolina were sampled for a study in August 2011. Standard places like cutting boards, kitchen counters, door handles, toilet seats and pillowcases were sampled. The bacterial families with the highest relative abundances across all of the collected samples were the Streptococcaceae (8.9%) (gram-positive), Corynebacteriaceae (5.6%) (gram-positive), and Lactobacillaceae (5.1%) (gram-positive). Since these are all gram-positive, their survival would also depend upon the acidity and nature of the surface. Keeping the humidity in the home in the sweet range of 40-60% will favor the production of more bacteria-killing hydroxyl radicals, and cleaning regularly is important. Wet, dusty or cluttered surfaces will actually promote good bacteria survival, but they also promote bad bacteria survival too, so to play it safe, it’s best to keep surfaces clean!